REGRANEX®
(becaplermin) Gel, 0.01%
A recombinant platelet-derived growth factor (PDGF) therapy transforming care for lower extremity diabetic neuropathic ulcer.
REGRANEX (becaplermin) Gel, 0.01% is a product of:



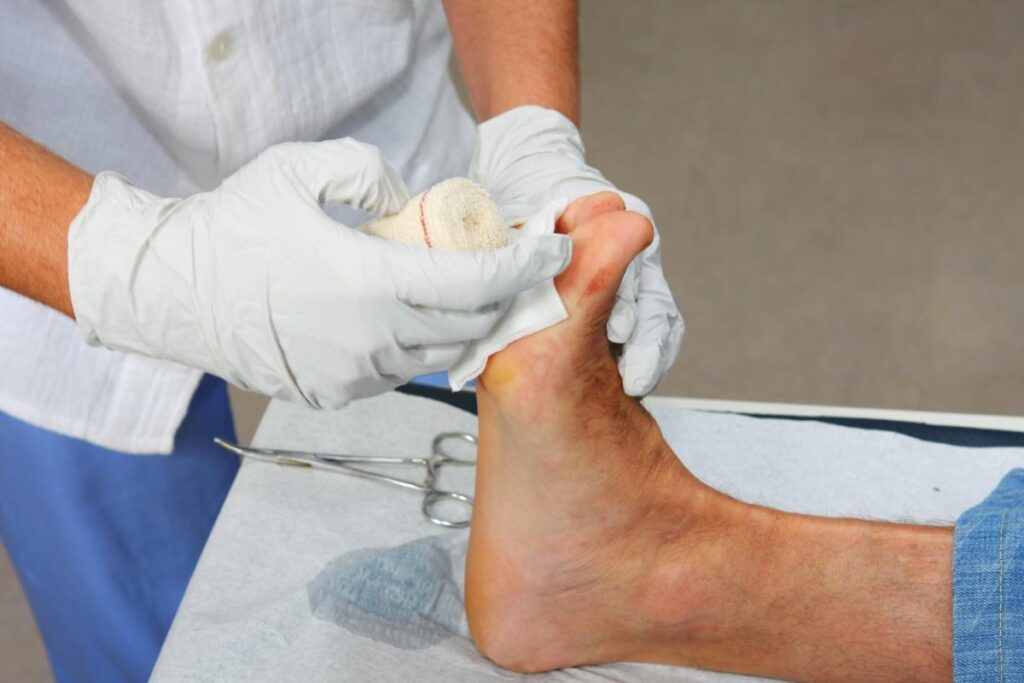
As rates of diabetes continue to increase, so does the frequency of diabetic foot ulcer (DFU).
An estimated 15% of patients with diabetes are likely to develop a DFU, and 85% of diabetes-related lower extremity amputations are preceded by this condition.
REGRANEX Gel is the only FDA-approved PDGF for the treatment of diabetic neuropathic ulcers, formulated to act as a first-line treatment in conjunction with good ulcer care.
Engineered for growth and healing
REGRANEX’s recombinant pure PDGF formulation initiates the DFU healing process by attracting repair cells to the wound with targeted precision.

The REGRANEX Healing Process
REGRANEX stimulates tissue growth by promoting fibroblast proliferation, while also enhancing the rate of re-epithelialization and revascularization to facilitate body restoration.

An easy, three-step application process
REGRANEX is designed for a flexible, once-daily application schedule as part of effective wound care and assessment, to be applied by either the clinician, caregiver, or patient. Simply prepare, apply, and cover.
REGRANEX gel should be refrigerated when not in use.
Important Safety Information
INDICATION
REGRANEX® (becaplermin) gel 0.01% is indicated for the treatment of lower extremity diabetic neuropathic ulcers that extend into the subcutaneous tissue or beyond and have an adequate blood supply, as an adjunct to, and not a substitute for, good ulcer care practices.
Limitations of Use:
Efficacy of REGRANEX has not been established for the treatment of pressure ulcers or venous stasis ulcers, or on exposed joints, tendons, ligaments, and bone in humans. Not intended for use in wounds that close by primary intention.
For topical use; not for oral, ophthalmic, or intravaginal use.
CONTRAINDICATIONS
REGRANEX is contraindicated in patients with known neoplasm(s) at the site(s) of application.
WARNINGS & PRECAUTIONS
Risk of Cancer. REGRANEX contains becaplermin, which promotes cellular proliferation and angiogenesis. Malignancies distant from the site of application have been reported in both a clinical study and in postmarketing use.
Application Site Reactions. If application site reactions occur, consider the possibility of sensitization or irritation due to parabens or m-cresol. Further evaluate, interrupt or discontinue treatment, as appropriate.
ADVERSE REACTIONS
In clinical trials, erythematous rashes occurred in 2% of subjects treated with REGRANEX (and good ulcer care) or placebo (and good ulcer care). In a retrospective follow-up study, 8 of 291 subjects (2.7%) from the REGRANEX group and 2 of 200 subjects (1%) from the placebo group were diagnosed with cancers during the follow-up period, a relative risk of 2.7 (95% CI).
This risk information is not comprehensive. For complete product information, please see the full Prescribing Information.